

No specific size range of monomers exist in the literature because monomers of different categories or even within the same category can vary in size. In this article, we will discuss monomers in terms of their size, classification, structures, chemical combinations, their occurrence, and several other facts. The identical monomers join together via different types of chemical bonding to form giant-molecules called polymers. They are the smallest form of stable pure substance that can be joined together to form giant molecules or macromolecules. Monomers belong to the category of micro-molecules. They can be either macro-molecules or macro-molecules. Molecules are defined as the stable pure particles formed by the chemical combination of two or more atoms. In order to completely understand the concept of monomers, let us first revise our definition of molecules. Monomers can be defined as small molecules that join together to form larger molecules.
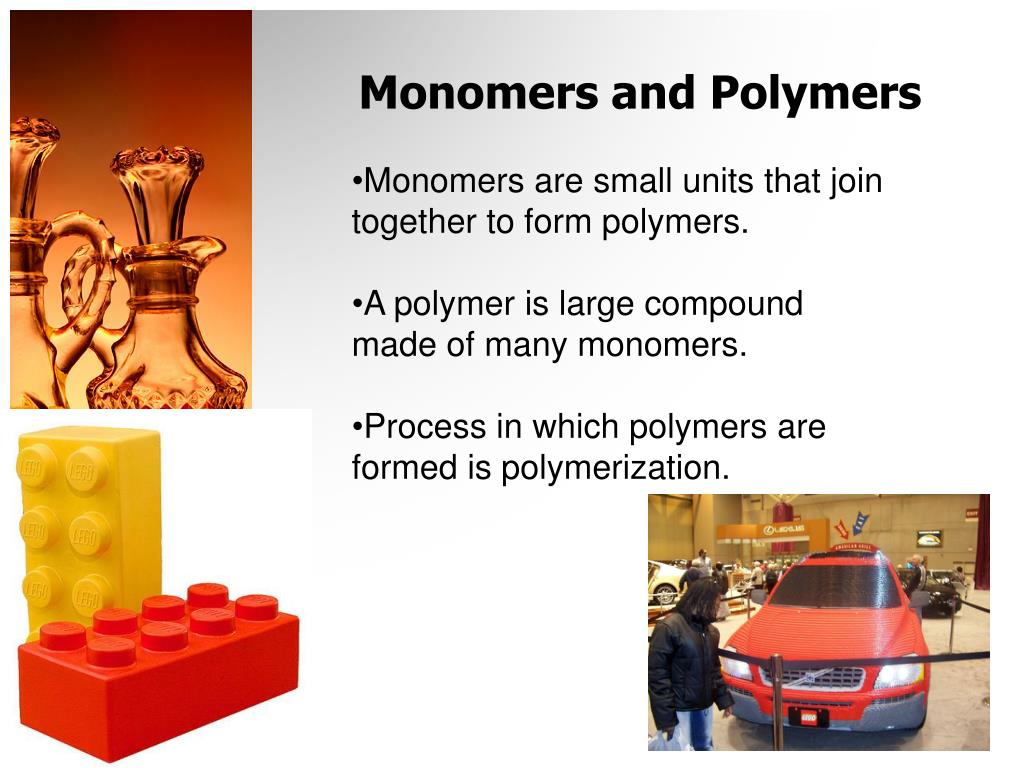
When there are zigzag lines with no atom in sight (/\/\/\), this means that carbons and hydrogens are the only connecting atoms. Why is everything so straight and awkward-looking? Fine, we will fix it. On the chemical bond level of things, here is what happens when your body makes a triglyceride.įirst, here's what one dehydration reaction looks like: Need a picture? We were thinking that, too. The name of this whole process is dehydration synthesis because monomers are literally coming together and synthesizing a polymer by dehydrating, or removing a water molecule. This blissful union is presided over by an enzyme, which is mainly there to help speed things along. (We add a dash to molecular groups, to show that they are attached to something else.) Having bonded, our lonely monomers are now a single polymer. While this is happening, the two monomers are binding to each other where they were bound to their respective hydrogen (–H) or hydroxyl (–OH) groups. Just when you think they’re going to start line dancing, a hydrogen (H) from one monomer binds with a hydroxyl group (OH) from the other monomer, and voilà! A water molecule is born: H + + OH - = H 2O. The process begins when two monomers line up next to each other. There is a process by which this joining usually occurs, and it's called dehydration synthesis. We would call it "Monamours." Clever, no? Anyway. How does this "linking together" happen? Are we talking about one monomer grabbing another monomer’s little molecular hand and never letting go? Not exactly, though that would make for an adorable webcomic. When a bunch of monomers join together into a much larger molecule, they form a polymer, meaning "many units" ( poly = many). In the molecular world, the small subunits that ultimately link together to form larger molecules are called monomers, which literally means "single unit" ( mono = one). Or if quilts aren't your thing, think of it like trying to find out who among your Facebook friends knows a guy who knows a guy who knows a guy who can introduce you to Matthew Lewis. Picture it like piecing together patches of a quilt.
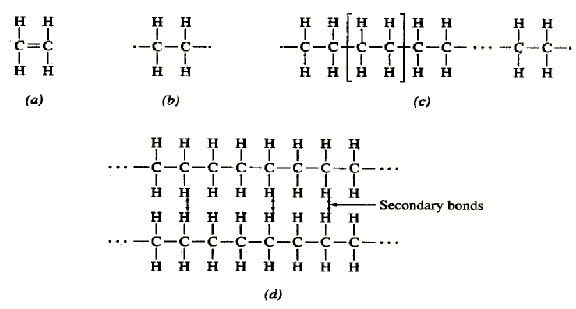
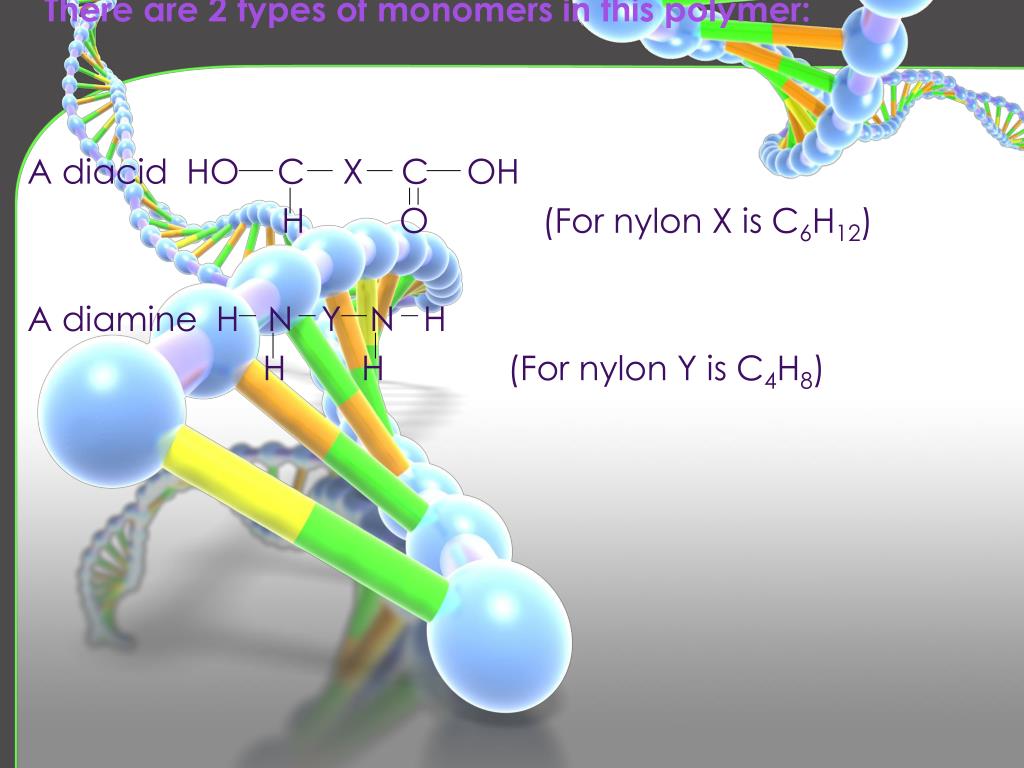
If you were trying to correctly assemble a molecule that big, you would probably want to start by putting together some smaller fragments and then carefully link those fragments together at the end. Others are large and unwieldy and can contain hundreds or thousands of atoms. Some biological molecules are relatively small and may contain a handful of atoms bound together. Monomers, Polymers, and Dehydration Synthesis


 0 kommentar(er)
0 kommentar(er)
